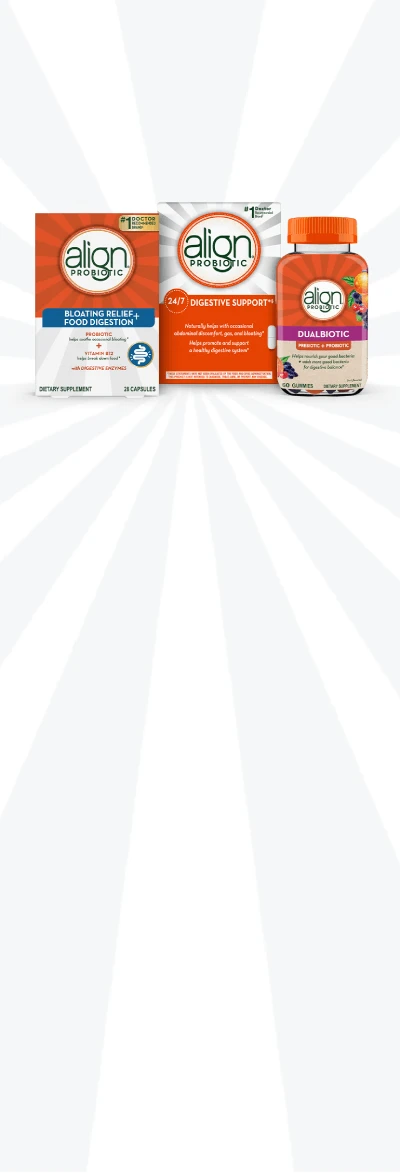
Ready to find your Align probiotic?
Align has a variety of products for everyone in the family - from probiotic capsules to prebiotic + probiotic gummies.
Align Probiotic 24/7 Digestive Support Capsules
Align 5X Extra Strength Probiotic Capsules
Bloating Reief + Food Digestion Capsules
Women's Dual Action Probiotic Supplement